 Ozone
Layer Depletion: Introduction
Ozone
Layer Depletion: Introduction Ozone
Layer Depletion: Introduction
Ozone
Layer Depletion: IntroductionSunlight contains some ultraviolet light, and when we expose ourselves to too much of it, we get a sunburn. Over time, too much exposure to ultraviolet light can lead to cataracts and skin cancer. The earth has a layer in the upper atmosphere, consisting mostly of ozone gas, that filters out most of the ultraviolet in the sun's radiation. Recently there has been scientific evidence that we have been releasing gases that damage this layer. Our country and others have reacted by invoking legislation that should eliminate these contaminants from the atmosphere.
http://core.ecu.edu/phys/spraguem/environment/ozone/o3_01.html
This page is a supplement to Mark
Sprague's Physics and the Environment, Phys 1050(1), class at East
Carolina University.
All About Ozone
Ozone
is a relatively simple molecule, consisting of three oxygen atoms bound
together. Yet it has dramatically different effects depending upon its location.
Near Earth's surface, where ozone comes into direct contact with life forms, it
primarily displays a destructive side. Because it reacts strongly with other
molecules, large concentrations of ozone near the ground prove toxic to living
things. At higher altitudes, where 90 percent of our planet's ozone resides, it
does a remarkable job of absorbing ultraviolet radiation. In the absence of this
gaseous shield in the stratosphere,
the harmful radiation has a perfect portal through which to strike Earth.
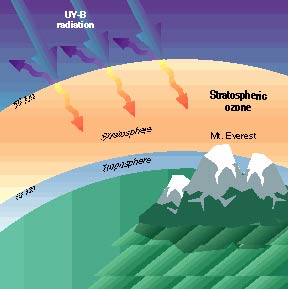
Stratospheric ozone occupies the region of the atmosphere
between 10 and 50 kilometers from the earth's surface and provides a shield
against damaging ultraviolet radiation.
http://www4.nationalacademies.org/beyond/beyonddiscovery.nsf/web/ozone3
A concerted global effort has been made to
protect the earth's ozone layer — our planet's fragile sunscreen.
 Production of
the industrial chemicals which once posed a major threat to the ozone layer has
been greatly reduced, and levels of some of these chemicals are now beginning to
decline in the lower atmosphere.
Production of
the industrial chemicals which once posed a major threat to the ozone layer has
been greatly reduced, and levels of some of these chemicals are now beginning to
decline in the lower atmosphere.
The ozone layer is expected to eventually recover, if all
nations maintain their efforts to reduce ozone-destroying chemicals. However, it
will probably be more than a decade before we begin to see definite signs of a
recovery, and at least the year 2050 before any substantial recovery occurs. At present, the layer is still thinning, especially at the
earth's poles. The "hole" over the Antarctic is continuing to
grow and considerable depletions are occurring in the Arctic. 
Mid-latitude areas, such as southern Canada and the U.S., are
also still
experiencing ozone
thinning.
Canada has played a key role in protecting the ozone layer. Our
nation was instrumental in the development of the Montreal Protocol, the
international agreement to reduce ozone-destroying chemicals. Canada is also a
leader in the scientific research which guides international action to protect our fragile skies.
Yet, we still have much to do - we must find ways to reduce the
use of the remaining industrial chemicals that continue to damage the ozone
layer, and to guard against any new threats. Scientists need to learn more about
ozone depletion, and how other stre sses on the environment, such as climate change, are affecting
the upper atmosphere.
sses on the environment, such as climate change, are affecting
the upper atmosphere.
http://www.msc-smc.ec.gc.ca/cd/factsheets/ozone/index_e.cfm
![]()
Stratospheric Ozone Depletion
This is a resource file for both teachers and students interested in the ozone layer. Stratospheric ozone depletion is a concern because the ozone layer in the stratosphere keeps 95-99% of the suns ultraviolet radiation from striking the earth. In this file are:
1.
Internet
resources for both ozone information, and neat digitized images
of the antarctic ozone hole.
2.
Printed
information including phone and e-mail contacts for those who wish to pursue
the issue of ozone depletion further. For those in a hurry, my favorite source
of quick yet reliable answers is Frequently-asked-Questions on Ozone by Robert
Parson. For general science questions on the atmosphere the college undergrad
textbook Atmospheric
Change: An Earth System Perspective, an authoritative text accessible to
those with a high school science background.
3.
An important part of the ozone discussion is the use of scientific
models in an effort to better understand the problem and make useful
predictions. I have written a laymans guide to scientific modelling which can
aid you in understanding some of the applications and limitations of modeling.
4.
Some
of the controversies surrounding the science and theories of ozone depletion
are summarized here.
5.
Brief
glossary of some of the terms and concepts used when discussing ozone
depletion.
6.
A summary of the ozone depletion issue, below, with highlighted terms
that you can click on for further information.
Stratospheric ozone depletion is a concern because the ozone layer in the stratosphere keeps 95-99% of the suns ultraviolet radiation from striking the earth. A number of consequences can result from increased levels of UV(ultraviolet radiation) striking the earth, including: genetic damage, eye damage and damage to marine life. Increased UV radiation in the lower atmosphere, called the troposphere, can result in increased amounts of photochemical smog. Photochemical smog is already a health hazard in many of the world's largest cities.
The decrease of stratospheric ozone was first reported in 1974 and the decrease was quickly linked to the increasing presence of a class of manmade compounds called CFC's or Chlorofluorocarbons. Many countries of the world have moved to reduce the use of CFC's but because of the slow rate of air mixing between the lower and upper atmosphere it is theorized that stratospheric CFC's will stay at a significant level well into the next century.
Stratospheric ozone depletion has become very much a controversial political and economic issue as well as a complex scientific issue. Major and minor sources of chlorine, and factors which affect ozone levels are still being sorted out among a great deal of media-generated excitement and misinformation; but the link between CFC's and Ozone depletion, and the major factors creating the antarctic ozone hole, are considered by most researchers to be well established facts. Scientific models of the atmosphere are being constructed in order to assist scientists in looking for other factors in Ozone depletion, evaluate their importance and predict what may happen to our atmosphere in the future.
**This file may be copied, distributed and archived but all copies must include this caveat as well. Caveat: This document is a compilation of information from a number of sources, with the gracious assistance of NASA and the IISME program, but does not necessarily reflect their views and definitely does not reflect the breadth of their knowledge. I am solely responsible for what is written here, including errors. Students should note that since this is not a peer reviewed publication, it should not be used as a reference for science projects; instead all scientifc facts and data should be referenced back to the original authors.
Brien Sparling. Chemistry Teacher. Live Oak High School. Morgan Hill. California. 95037
Curator: Jill Dunbar Last Update: May 30, 2001 NASA Official: William J Feiereisen
http://www.nas.nasa.gov/About/Education/Ozone/
NASA Advanced Supercomputing Division
Additional Resources:
 http://www.soton.ac.uk/~engenvir/environment/air/oz_intro.htm
http://www.soton.ac.uk/~engenvir/environment/air/oz_intro.htm
| Information about the Energy Commission, its programs, proceedings, and statistics about energy production and use in California. | Everything there is to know about energy efficiency for the home or business, information about alternative fuel vehicles and buying an energy efficient vehicle, and information about renewable energy. |
|
|
Report Bad Hyper
Links Here |