
Adjustments to Dalton's Atomic Theory
We now know that atoms of the same element can have different masses and are called isotopes. Isotopes have a different number of neutrons than the "average" atom of an element.
We have also discovered the possibility of atomic fission; splitting the atom (Hiroshima & Nagasaki).
Def: An atom is the smallest unit of a
substance that retains the properties of that
substance.
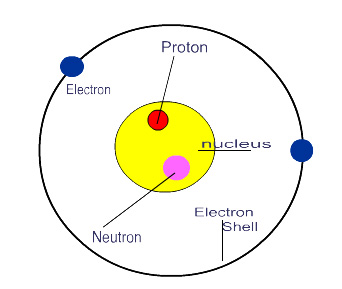
Atoms are composed of three types of particles:
|
Particles |
Symbol |
Charge |
Location |
|
Protons |
p+ |
Positive
charge |
Found
in the nucleus |
|
Neutrons |
N0 |
Neutral
(no) charge |
Found
in the nucleus |
|
Electrons |
e- |
Negative
charge |
Found
orbiting the nucleus |
Nucleus – The positively charged central region of an atom, composed of protons and neutrons and containing almost all of the mass of the atom. More simply the nucleus is the “middle part” of an atom defined by an imaginary boundary separating it from the orbiting electrons.
Rutherford's view of the atom (1914)
http://www.daviddarling.info/encyclopedia/R/Rutherfords_experiment_and_atomic_model.html
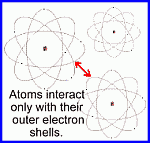
http://www.worsleyschool.net/science/files/rutherford/atom.html
Bohr models of various atoms. (1920)
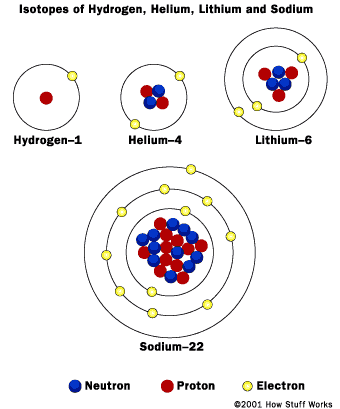
http://www.clickandlearn.org/Gr9_Sci/atoms/modelsoftheatom.html
Quantum model of a sodium atom. (Current)
Atomic Number- Determined by the number
of protons
Atomic Mass Unit- mass of one proton or one neutron (they are about the same)
Atomic Mass- Mass of an atom:
determined by adding the protons and neutrons
(Actually this number is an
average; re. isotopes)
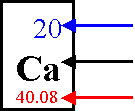 Atomic Number
Atomic Number
Atomic Symbol
Atomic
Mass
The number of protons is equal to the number of
electrons
p+ = e-
The mass number minus the atomic number equals the
number of neutrons
mass # - atomic # = N0
Orbital- three-dimensional region about the nucleus in which a particular electron can be found.
Quantum numbers- numbers that specify the properties of atomic orbitals and of their electrons.
Principal Quantum number- indicates the main energy levels surrounding a nucleus, sometimes referred to as shells or orbitals. (1,2,3,4,5,6,7) Larger numbers are farther away from the nucleus and electrons found here have more energy.
Orbital quantum number- indicates the shape of an orbital. Often referred to as sublevels or subshells. (s,p,d,f in order of increasing energy)
s orbitals can hold 2
electrons
p orbitals can hold 6
electrons
d orbitals can hold 10
electrons
f orbitals can hold 14
electrons
Magnetic quantum number- indicates the orientation of an orbital about the nucleus. (x,y.z coordinates)
Spin quantum number- indicates the spin of an electron (+1/2, or -1/2)
Atoms are mostly empty space. If the nucleus of an atom had the diameter of a dime, the outer edge of the atom would be about 50 yards away.
If a proton were enlarged to the size of the head of a pin, the
outer edge of the atom would be about 100 meters away.

If this picture were drawn to scale:
And if the protons and neutrons were a centimeter in diameter;
Then the electrons would be less than the diameter of a hair; and
The entire atom's diameter would be greater than the length of 30 football
fields!
If an atom were as large as a football stadium, the nucleus would be the size of a small ladybug crawling across the 50-yard line. In spite of this size difference, virtually an of the mass of an atom is concentrated in its nucleus. One electron, which has a negative charge, weighs only 1/1836 as much as the lightest of all nuclei, that of the hydrogen atom (proton).
In addition, all the particles (protons, neutrons, and electrons) are constantly
in motion.
Rutherford's Gold Foil Experiment
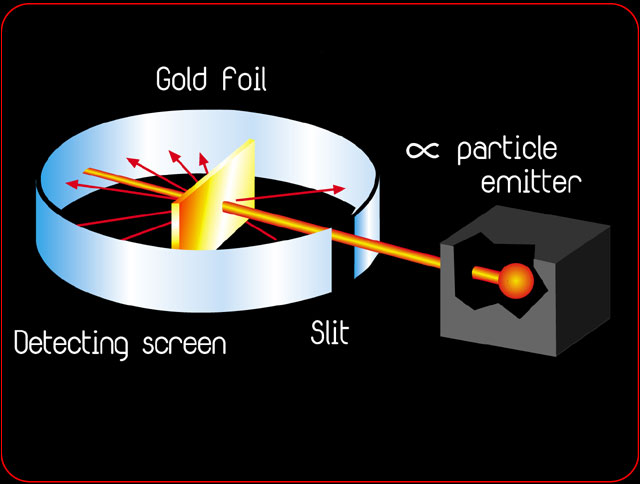
http://www.rsc.org/chemsoc/timeline//pages/1911.html
1911- Ernest Rutherford publishes his atomic theory describing the atom as having a central positive nucleus surrounded by negative orbiting electrons. This model suggested that most of the mass of the atom was contained in the small nucleus, and that the rest of the atom was mostly empty space. Rutherford came to this conclusion following the results of his famous gold foil experiment. This experiment involved the firing of radioactive particles through minutely thin metal foils (notably gold) and detecting them using screens coated with zinc sulfide (a scintillator). Rutherford found that although the vast majority of particles passed straight through the foil approximately 1 in 8000 were deflected leading him to his theory that most of the atom was made up of 'empty space'.
What percentage of the volume of an atom is just empty space?
If you know of some information you think should be included on this page, please let me know!!
Interactive Periodic Charts:
![]() http://www.chemicalelements.com/groups/halogens.html
http://www.chemicalelements.com/groups/halogens.html
http://www.ktf-split.hr/periodni/en/
go on to learn about the Interaction of Matter
Sources:
http://nwscc.cc.al.us/distance/bio103/topic3/3/sld001.htm A good PowerPoint presentation about atoms
http://www.colorado.edu/physics/2000/waves_particles/wavpart2.html cool particle applet
http://www.howstuffworks.com/framed.htm?parent=atom.htm&url=http://chipo.chem.uic.edu/web1/ocol/SB/1-2.htm more info on orbitals & shapes
http://www.chemguide.co.uk/atoms/properties/gcse.html#top A SIMPLE VIEW OF ATOMIC STRUCTURE
 http://www.biologylessons.sdsu.edu/classes/lab2/index.html
http://www.biologylessons.sdsu.edu/classes/lab2/index.html
http://press.web.cern.ch/pdg/cpep/big_question.html how do atoms/molecules "stick" together
http://neon.chem.ox.ac.uk/vrchemistry/AMM/HTML/page01.htm an excellent tutorial with movies & nice graphics
http://chem.lapeer.org/Chem1Docs/ElementQuiz.html online elements quiz
More Sites about atoms:
The Atom - Succeed in Physical Science
http://lpmpjogja.diknas.go.id/kc/a/atom/atom.htm
|
Report Bad Hyper
Links Here |